Details of the Drug
General Information of Drug (ID: DMG2SKR)
| Drug Name |
Tedizolid
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Tedizolid; Torezolid; 856866-72-3; TR-700; Da 7157; UNII-97HLQ82NGL; DA-7157; TR 700; 97HLQ82NGL; CHEBI:82717; (5R)-3-[3-fluoro-4-[6-(2-methyltetrazol-5-yl)-3-pyridyl]phenyl]-5-(hydroxymethyl)oxazolidin-2-one; Tedizolid [USAN:INN]; Tedizolid (USAN/INN); SCHEMBL440398; CHEMBL1257051; DTXSID10234975; XFALPSLJIHVRKE-GFCCVEGCSA-N; MolPort-042-624-220; AI025; BCP02830; ZINC43100956; AKOS025401974; CS-0687; NCGC00379072-02; AN-27109; AK322133; AC-27738; BC600578; HY-14855
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
EnterococcusStreptococcusStaphylococcus
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
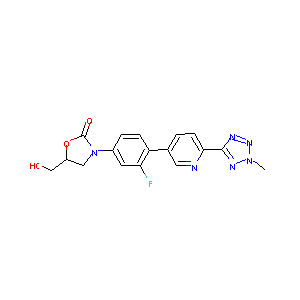 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 370.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Tedizolid (Comorbidity)
|
|||||||||||||||||||||||||||||||||||
References
